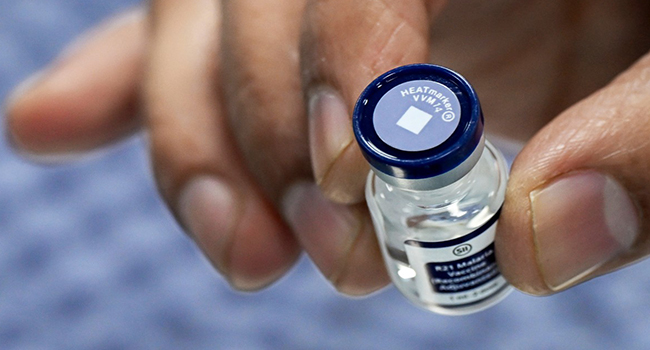
The WHO Regional Office for Africa said in a statement on Monday that Ghana, Kenya, and Malawi will take part in the pilot programme which will take off in 2018.
According to the statement, the injectable vaccine, RTS,S, was developed to protect young children from the most deadly form of malaria caused by Plasmodium falciparum.
WHO Regional Director for Africa, Dr Matshidiso Moeti, was quoted as saying, “The prospect of a malaria vaccine is great news. Information gathered in the pilot will help us make decisions on the wider use of this vaccine.
“Combined with existing malaria interventions, such a vaccine would have the potential to save tens of thousands of lives in Africa.”
The pilot programme is meant to assess whether the vaccine’s protective effect in children aged 5–17 months old during Phase III testing can be replicated in real life.
Specifically, the pilot programme will assess the feasibility of delivering the required four doses of RTS,S, the vaccine’s potential role in reducing childhood deaths, and its safety in the context of routine use, WHO said.
The vaccine, RTS,S, was developed by GSK and is the first malaria vaccine to have successfully completed a Phase III clinical trial.
The trial was conducted between 2009 and 2014 through a partnership involving GSK, the PATH Malaria Vaccine Initiative (with support from the Bill & Melinda Gates Foundation), and a network of African research sites in seven African countries—including Ghana, Kenya, and Malawi.
WHO explained that it chose the three countries to participate in the pilot programme based on high coverage of long-lasting insecticidal-treated nets; well-functioning malaria and immunisation programmes, a high malaria burden even after scale-up of LLINs, and participation in the Phase III RTS,S malaria vaccine trial.