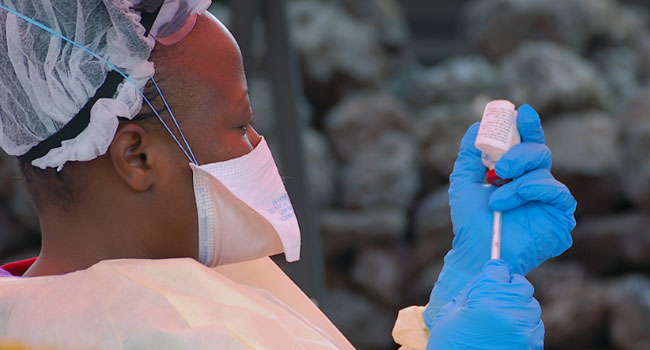
The Democratic Republic of Congo will introduce a second Ebola vaccine next month, the World Health Organization said Monday, as a top medical charity accused the UN agency of rationing doses of the main drug to protect against the disease.
DRC’s latest Ebola epidemic, which began in August 2018, has killed more than 2,100 people, making it the second deadliest outbreak of the virus, after the West Africa pandemic of 2014-2016.
Ebola fighters have been hindered by chronic insecurity in the affected provinces of eastern DRC, but much of the controversy surrounding the response has centred on the use of vaccines.
READ ALSO: Mugabe Died Of Cancer, Says Zimbabwe Media
More than 223,000 people living in active Ebola transmission zones have received a vaccination produced by the pharma giant Merck.
The WHO has for month been pushing Kinshasa to approve the use of a second experimental product, made by Johnson & Johnson, to protect those living outside of direct transmission zones.
The J&J vaccine had been rejected by DRC’s former health minister Oly Ilunga, who cited the risks of introducing a new product in communities where mistrust of Ebola responders is already high.
But Ilunga’s resignation in July appeared to pave the way for approval of the second vaccine.
In a statement issued on Monday, the health ministry said the second vaccine would be used as a “preventive” measure, given the risk that stocks of the Merck vaccine could run out if the epidemic persists.
The ministry said it had scrutinised candidate Ebola vaccines, and “the Johnson & Johnson vaccine has most scientific data… it is not toxic and can provide protection.”
“This vaccine presents no danger to the public,” the statement said, adding that the first priority would be to use it to inoculate “Congolese traders who regularly visit Rwanda.”
“We must also protect Rwanda”, it said.
The WHO said in a statement that DRC planned to introduce the J&J product from “mid-October.”
“This vaccine, which is given as a 2-dose course, 56 days apart, will be provided under approved protocols to targeted at-risk populations in areas that do not have active Ebola transmission as an additional tool to extend protection against the virus.”
WHO Director-General Tedros Adhanom Ghebreyesus praised the latest decision by DRC authorities, who he said “have once again shown leadership and their determination to end this outbreak as soon as possible”.
– ‘Rationing’? –
Doctors Without Borders, which has repeatedly criticised WHO’s leadership of the Ebola response, levelled fresh criticism against the agency on Monday.
“One of the main problems currently is the fact that, in practice, the (Merck) vaccine is rationed by the WHO and that too few people at risk are protected today,” the charity known by its French acronym MSF said in a statement.
In an interview with AFP in July, MSF’s international president Joanne Liu called on WHO to vaccinate whole villages where Ebola cases had emerged, rather than simply targeting the contacts of those infected.
The charity also renewed its complaints over “the opaque management of the vaccine supplied by the World Health Organization.”
“It’s like giving firefighters a bucket of water to put out a fire, but only allowing them to use one cup of water a day,” Natalie Roberts, MSF’s emergency coordinator,” said in a statement.
“Merck recently stated that in addition to the 245,000 doses already delivered to WHO, they are ready to ship another 190,000 doses if required and that 650,000 additional doses will be available over the next six to 18 months,” MSF said.
The charity also called for the creation of “an independent international coordination committee” to “guarantee the transparency of the management of stocks and data sharing”.
– ‘Everything possible’ –
The WHO denied limiting the availability of the vaccine, saying it was doing “everything possible” to end the epidemic.
“Along with the DRC government, no-one wants to bring this epidemic to an end more than WHO,” the agency’s emergency director, Mike Ryan, said in a statement.
The WHO is “not limiting access to the vaccine but rather implementing a strategy recommended by an independent advisory body of experts,” Ryan added.
The agency said last week that as of September 17, DRC had registered a total of 3,145 cases of Ebola since the outbreak began over a year ago, including 2,103 deaths.
It has declared the Ebola epidemic a “public health emergency of international concern”, a rare designation used only for the gravest epidemics.