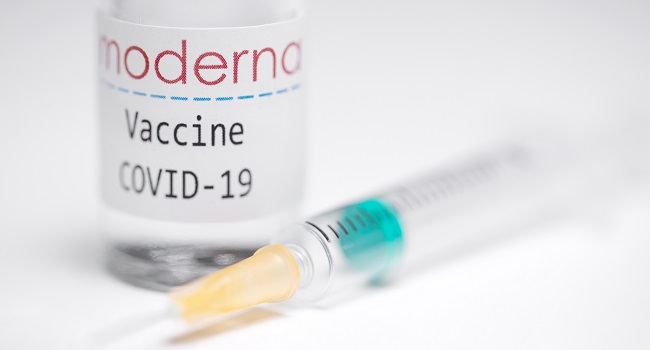
The EU’s medicines watchdog said on Thursday that it had brought forward the date for a decision on authorising Moderna’s coronavirus vaccine by nearly a week to January 6.
The Amsterdam-based European Medicines Agency has been under growing pressure to speed up, and earlier this week accelerated the timeframe for Pfizer/BioNTech’s jab.
The EMA had originally been due to decide on US-based Moderna’s vaccine on January 12, but said the company had submitted extra data on Thursday “ahead of schedule”.
“Taking due account of the progress made, the Committee has scheduled an extraordinary meeting on 6 January 2021 to conclude its assessment, if possible,” the EMA said, referring to the medicines committee that decides on such issues.
“The meeting planned for 12 January 2021 will be maintained if needed.”
A clinical trial of 30,400 people found the Moderna vaccine was 94.1 percent effective in preventing Covid-19 compared to a placebo, performing slightly better in younger adults compared to the elderly.
EMA chief Emer Cooke said the under-fire agency had shown it was able to respond.
“We have been able to revise the timetables for the evaluation of the Covid-19 vaccines due to the incredible efforts of everybody involved in these assessments,” Cooke said in a statement.
“The number of infections is increasing across Europe and we are aware of the huge responsibility we have to get a vaccine to the market as quickly as is feasible, whilst maintaining the robustness of our scientific review.”
READ ALSO: US To Revise COVID-19 Vaccine Guidance After Allergic Reactions
With Britain and the United States having pushed through emergency authorisation of the Pfizer/BioNTech vaccine, the EMA has been under pressure to speed up, notably from Germany.
The watchdog, which moved from London last year after Brexit, will decide on a year-long “conditional marketing authorisation” for Pfizer/BioNTech on Monday, having brought the date forward from December 29.




