Graeme Robertson / AFP / POOL
Britain on Wednesday became the first nation to approve the coronavirus vaccine developed by AstraZeneca and the University of Oxford, as Germany logged its highest daily death toll with the pandemic surging worldwide.
Fears have also grown following the detection in Britain of a new strain of the virus experts suspect is more contagious, and the variant has been found in a number of other countries, including the United States and India.
A year after the World Health Organization first mentioned a pneumonia in China later identified as Covid-19, the virus has killed more than 1.79 million, devastated the global economy and driven the race for a vaccine to halt the pandemic.
International efforts helped develop vaccines in record time, and following Pfizer-BioNTech and Moderna, the AstraZeneca-Oxford candidate became the third to win approval in the Western world.
“We will now move to vaccinate as many people as quickly as possible,” tweeted British Prime Minister Boris Johnson.
Unlike the Pfizer-BioNTech and Moderna vaccines, the one from AstraZeneca and Oxford does not need to be stored at very low temperatures.
It can be kept, transported and handled at normal refrigerated conditions, making it easier and cheaper to administer, which is particularly important for less wealthy nations.
It is unlikely to get approval from the European Union until at least a month however.
Noel Walthion, deputy executive director of the European Medicines Agency told Belgium’s Het Nieuwsblad newspaper that a possible approval in January was “unlikely,” an agency statement confirmed.
Russian, Chinese vaccines
Russia and China also claim to have developed Covid-19 vaccines, and have already started administering them.
Chinese pharma giant Sinopharm on Tuesday said Phase 3 trials of its candidate had shown 79-percent effectiveness, short of the more than 90 percent achieved by Pfizer-BioNTech and Moderna. The firm has applied to China’s drug regulator for approval.
But Beijing has struggled to gain international trust for its vaccines, hindered by a lack of data transparency as well as criticism over its handling of the initial outbreak of the virus in the central Chinese city of Wuhan and its attempts to silence whistleblowers.
The number of infections in that city may have been 10 times higher than official figures suggest, according to a study by China’s Centre for Disease Control.
A leading scientist also criticised the French government for the slow roll-out of its vaccination programme.
France’s strategy “is not suited to a situation that is so dangerous,” said Axel Kahn, a prominent geneticist who leads the National League against Cancer.
New variant worries
Even as vaccinations ramp up in Europe and North America, global infections have surged to 82 million, with nearly 1.8 million deaths.
Germany, which had handled the first coronavirus wave relatively well, has been hit hard by the second. It logged more than 1,000 daily deaths for the first time, authorities said Wednesday.
Germany is under a partial lockdown, with most shops closed along with schools, restaurants, cultural and leisure facilities.
But with Chancellor Angela Merkel due to meet senior politicians to discuss the situation next Tuesday, some are already pressing to extend the existing lockdown beyond the current January 10 end-date.
Health Minister Jens Spahn told German broadcaster ARD the country was “nowhere near where we need to be” and “there will undoubtedly be measures” after January 10.
With England registering record daily figures for infections after the discovery of a new variant of the virus that experts believe could be more contagious, that same variant is now turning up in other countries.
Indian authorities were trying Wednesday to track down tens of thousands of recent arrivals from Britain as cases of the new variant more than doubled in 24 hours.
Health workers in the United States and Latin America detected it on Tuesday for the first time.
The EU health agency has warned the strain carries a high risk for more hospitalisations and deaths — not because the infections are more severe but because it spreads more easily.
‘Greatest operational challenge’
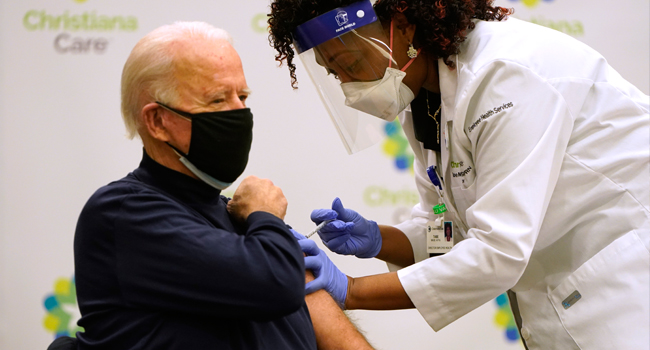
In the United States — the worst-hit nation in the world — President-elect Joe Biden called mass vaccination “the greatest operational challenge we’ve ever faced as a nation”.
The Trump administration had said that 20 million Americans would be vaccinated by the end of December, but so far just over two million have received the first shot of the vaccine, according to the Centers for Disease Control and Prevention.
Biden, who takes over from Donald Trump on January 20, renewed his promise to administer 100 million vaccine doses in his first 100 days in office, and confirmed he would invoke a Korean War-era law to force private industry to step up production.
“The Trump administration’s plan to distribute vaccines is falling far behind,” Biden said.
“I’m going to move Heaven and Earth to get us going in the right direction.”
But he warned: “The next few weeks and months are going to be very tough — a very tough period for our nation, maybe the toughest during this entire pandemic.”